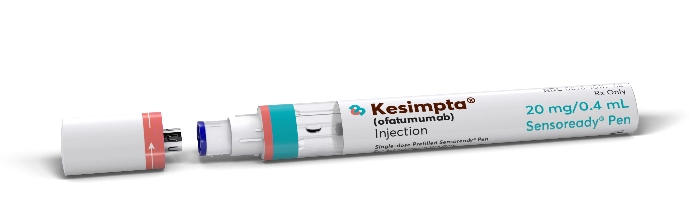
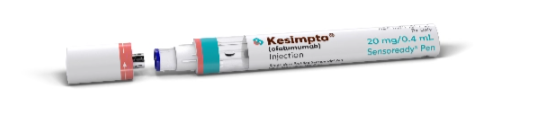
HOW THE KESIMPTA SENSOREADY® PEN WORKS

 1650611629 1_otxtm9yc
1650611629 1_otxtm9yc

 The idea of giving myself a shot, and
needles, in general, seemed pretty scary. But once I held the Sensoready®
auto-injector pen, I knew I could do it.
The idea of giving myself a shot, and
needles, in general, seemed pretty scary. But once I held the Sensoready®
auto-injector pen, I knew I could do it. 

– SARAH Q.
Sarah Q. has taken KESIMPTA and has been compensated for her time.



– SARAH Q.
Sarah Q. has taken KESIMPTA and has been compensated for her time.
MEET THE KESIMPTA SENSOREADY® PEN
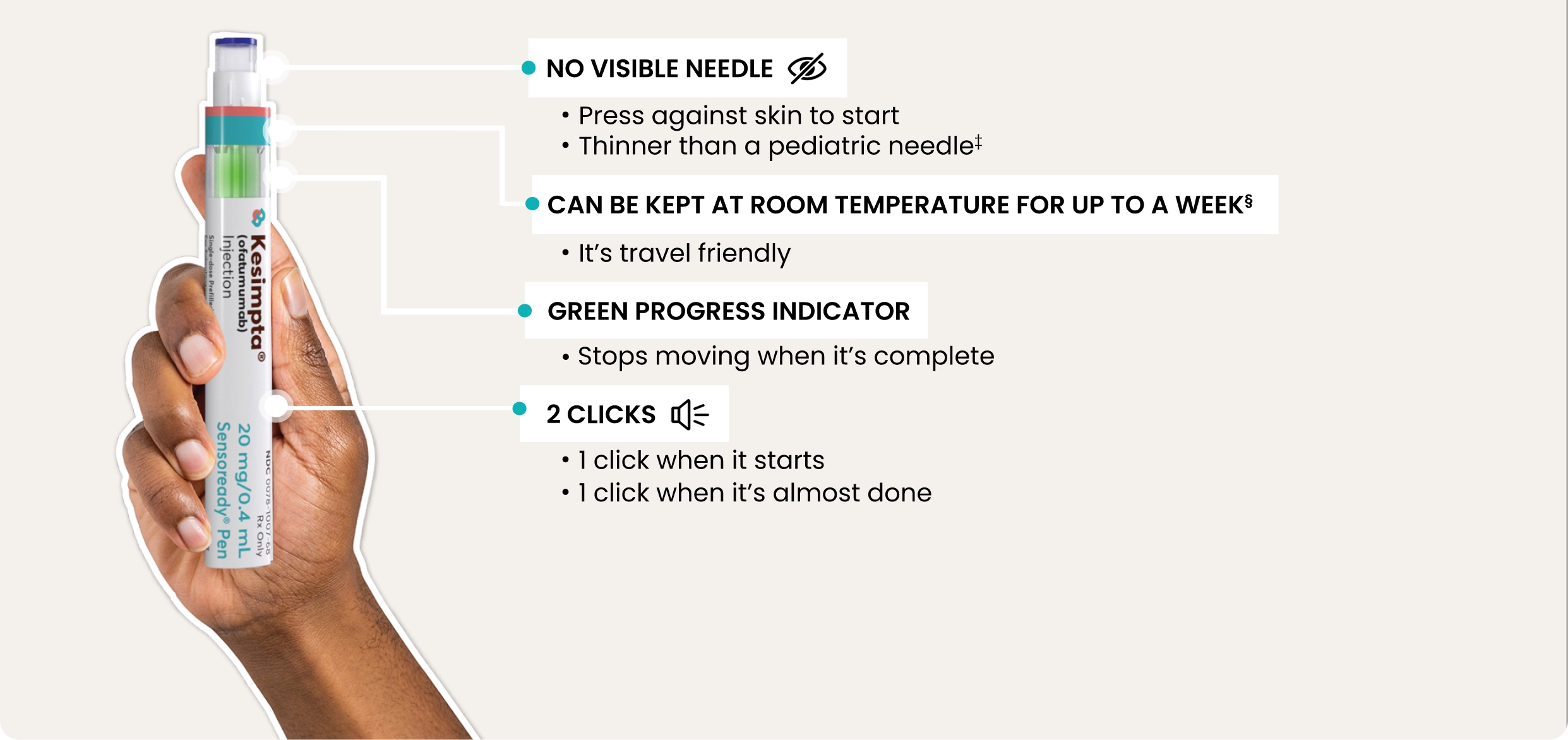
Ensure the progress indicator fills the window and stops moving
before removing the KESIMPTA Sensoready® Pen. Full
Instructions for Use.
§KESIMPTA Sensoready® Pens
must be refrigerated at 2 °C to 8 °C (36 °F to 46 °F). Keep in
original carton to protect from light until use. If necessary,
they may be stored at room temperature below 30 °C (86 °F) for
up to 7 days and returned to refrigerator, to be used within 7
days. If not used within those 7 days, discard the medicine.
Ensure the progress indicator fills the window and stops moving before removing the KESIMPTA Sensoready® Pen. Full Instructions for Use.
§KESIMPTA Sensoready® Pens must be refrigerated at 2 °C to 8 °C (36 °F to 46 °F). Keep in original carton to protect from light until use. If necessary, they may be stored at room temperature below 30 °C (86 °F) for up to 7 days and returned to refrigerator, to be used within 7 days. If not used within those 7 days, discard the medicine.



– MAGGIE S.
Maggie S. has taken KESIMPTA and has been compensated for her time.



– MAGGIE S.
Maggie S. has taken KESIMPTA and has been compensated for her time.
WHAT PEOPLE ARE SAYING
In a real-world survey,|| we asked people
taking KESIMPTA® to tell us what they thought about the
KESIMPTA
Sensoready® Pen. Here's what they had to
say:
Patients who take KESIMPTA
found the pen easy to use||:


||Real-world survey of 105 US patients (aged ≥18) who have been diagnosed with RMS for more than 1 year and who self-administered KESIMPTA using the Sensoready® Pen within the previous 12 months. Participants were asked 30 questions. Ratings were measured on a scale of 1-5, with higher scores indicating positive responses. 89.5% of patients scored a 4 or 5 on characteristics of overall ease of use and ease of monthly dosing schedule. Questionnaire has not been validated.
HERE'S HOW TO GET STARTED ON KESIMPTA DOSING
You'll give yourself 1 dose
per week for the first 3 weeks, and then you'll skip a week.
After that, you can move on to 1
dose per month.

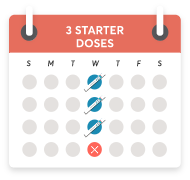
Pick a day and start with 1 dose a week for the first 3 weeks. Skip the 4th week.


Take your first monthly maintenance dose on the 5th week.


Continue taking KESIMPTA once a month.

TRACKING TIP
TRACKING TIP
To keep it simple, try to take KESIMPTA on the same date
every month.
EXAMPLE: If your first monthly dose is on April 15, take your next dose on May 15.

TRACKING TIP
To keep it simple, try to take KESIMPTA on the same date
every month.
EXAMPLE: If your first monthly dose is on April 15, take your next dose on May 15.
KESIMPTA & Other B-cell Treatments
With the KESIMPTA auto-injector pen, you can treat your RMS at
home in less than one minute a month, when
ready. Other
B-cell treatments are administered by infusion.


KESIMPTA is administered once a month subcutaneously after 3
weekly starter doses.
Ocrevus and Briumvi are dosed
twice yearly via infusion.
¶Typical administration time when ready to inject. Someone from your health care team will give you guidance on how to take KESIMPTA for the first time.
This is not a complete list of all the available treatments for RMS. The comparison pertains only to differences in dosing and administration and should not be considered a comparison of efficacy or safety. Please see each product's respective Prescribing Information for additional information including indication, dosing, and safety. Trademarks are the property of their respective owners.
¶Typical administration time when ready to inject. Someone from your health care team will give you guidance on how to take KESIMPTA for the first time.
This is not a complete list of all the available treatments for RMS. The comparison pertains only to differences in dosing and administration and should not be considered a comparison of efficacy or safety. Please see each product's respective Prescribing Information for additional information including indication, dosing, and safety. Trademarks are the property of their respective owners.
GET MORE TIPS FOR TAKING KESIMPTA
Someone from your health care team
will
give you guidance on how to use the
KESIMPTA
Sensoready® Pen for the
first
time. If you're nervous, you can receive your first dose at
your doctor's office.
Watch our Quick Tips video for tips and instructions on how to administer.
Get complete details about how to take KESIMPTA.
Learn about support and virtual training available through Alongside™ KESIMPTA.
Learn how you can pay
as little as $0 copay.#
Take a look at commonly asked questions.
GET SOME ANSWERS