MEET THE PEN

THE PEN FEATURES HELP YOU DO IT RIGHT
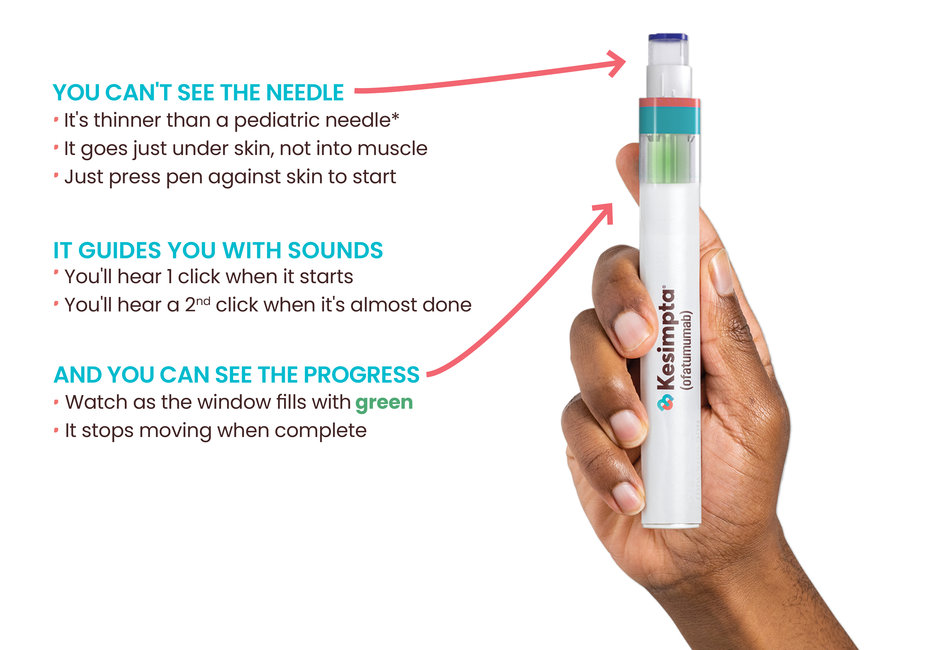
The KESIMPTA auto-injector pen has features that help you use it safely from the comfort of home. When you’re ready to start on KESIMPTA, someone from your health care team will show you how to use the pen so you’ll be ready on treatment day.
Ensure the green progress indicator fills the window and stops moving before removing the pen. Full Instructions for Use.
*KESIMPTA pens come with a staked needle of 29-gauge. Pediatric needles average between 23-gauge and 25-gauge.

Artist, MS Advocate
Switched to KESIMPTA: 2022
“The way the pen is designed puts the needs of people like me first.”
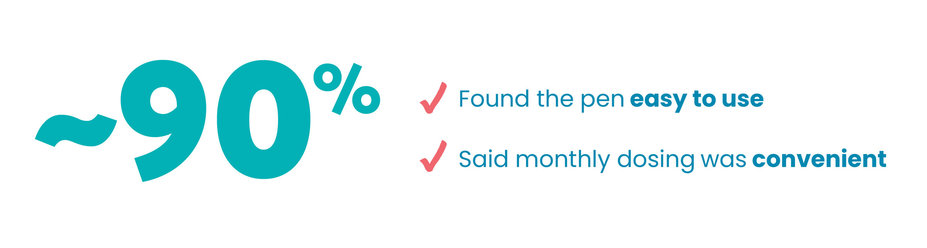
THE PEN IS EASY TO USE†
In a real-world survey,† people described their experience with the auto-injector pen. Here's what they had to say:

‡Based on a survey of MS nurses (N=50) and patients (N=80) in the US, Germany, France, and Italy. Participants were asked to compare attributes of the KESIMPTA auto-injector pen with those of other disease modifying therapy (DMT) auto-injectors, some of which are not available in the US. The KESIMPTA pen was not injected during the survey, nor were all devices compared against each other by participants. A total of 17 attributes were assessed, with “easy to perform self-injection with the pen,” “ease of preparation and set-up,” and “ease of training patient in use” among those most preferred.
GET STARTED WITH CONFIDENCE

Before starting KESIMPTA, someone from your health care team will show you how to take it. But if you’d like extra support to feel ready, more training resources are available here—and you can even get help with your first dose at your doctor’s office.

If you want, a training pen is available to help you practice before using the actual auto-injector pen.
The pen comes prefilled and ready to go when you are§—no premedications|| like steroids or 1st dose observation required.
||Only limited benefit of premedication with corticosteroids, antihistamines, or acetaminophen was seen in RMS clinical studies.
Up Next:
Discover how KESIMPTA can fit into your life.
Want more info to help you decide?
Find out why so many people are choosing KESIMPTA.
Prescribed KESIMPTA?
97% of all prescriptions filled have a $0 out of pocket cost when used with the Access Card.¶#
RMS, relapsing multiple sclerosis.
¶Limitations apply. Offer not valid under Medicare, Medicaid, or any other federal or state health insurance program. Patients with commercial insurance who are initially denied coverage may receive free KESIMPTA for up to 12 months while seeking coverage. Patients with commercial insurance who have coverage for KESIMPTA may receive up to $18,000 in annual co-pay benefits. Novartis reserves the right to rescind, revoke, or amend this program without notice. Additional limitations may apply. See complete Terms & Conditions at start.kesimpta.com.
#2024 data on file.